Acerca dos genéricos
Recuar a 2012:
Recuar a 2010:
"What Dingle needed, though, wasn’t a higher dose of new generic medicine, but the effectiveness of the brand-name drug. “When I went to pick up the new dose, the pharmacist took a minute to look at my prescriptions, then confirmed that my levels had changed after I switched from Synthroid to generic,” Dingle says. “He said he had seen variations like this, not only when the prescription changed from Synthroid to generics but also between batches of generics.”
...
The active ingredient of the generic must have the same pharmacokinetics as the original drug, which means it must behave the exact same way and reach the same maximum dose in the blood on the appropriate dosing. If the active ingredient is indeed bioequivalent, the drug will likely be equally effective and safe as the original.
But there may be a cost to the cheaper route. A drug’s inactive ingredients (binders, fillers, detergents, dyes, antioxidants, and sugars, which pharmacists call excipients) aren’t subject to the same level of regulation and research as active ingredients. While additional ingredients aren’t allowed to affect how the drug functions in the body and must be proven as “safe” to the FDA, those inactive ingredients can vary widely between brand names and generics — and between generics themselves.
...
For some patients, generic medications can cause a number of adverse reactions, meaning a drug can compound health concerns instead of alleviating them. This is often linked to a person’s biology; for example, a lactose-intolerant patient who switches to a generic drug with a lactose-based excipient might experience stomach issues, which could in turn decrease drug absorption.
...
Which leads to another drug-related phenomenon: the “nocebo” effect. Studies show using generic drugs can cause an anti-placebo effect of worsening symptoms or problematic side effects."


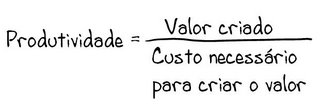







Sem comentários:
Enviar um comentário